By Karl Ernst Grund, Annette Zipfel. Institution: Experimental Surgical Endoscopy, Dept. Visceral and Transplant Surgery, University Hospital Tübingen, Germany
Correspondence to: Prof. Dr. med. K. E. Grund. Experimentelle Chirurgische Endoskopie. Zentrum für Medizinische Forschung. Waldhörnlestr. 22. D – 72072 Tübingen. [email protected]
The implantation of self-expanding metal stents (SEMS) into the colon – especially as a “bridge to surgery” – has become increasingly established, even though the characteristics of the colon make the implantation of prostheses difficult and no optimal stents for this site are available to date.
In acute colonic obstruction, stent placement allows an immediate decompression of the over-distended colon, and can help to avoid emergency surgery associated with high mortality and complication rate. Alternative methods, e.g. an endoscopically placed decompression tube, should be considered as well as oncologic aspects.
In spite of a large number of positive study results by stenting, a critical analysis of the publications based on various parameters reveals a considerable heterogeneity.
To yield good results, stent implantation in the colon should only be performed by an experienced endoscopist.
Key words: SEMS, colonic stent, colonic obstruction, decompression
Since the mid-1990s, the implantation of self-expanding metal stents (SEMS) into the esophagus and the biliary tree has increasingly become a standard procedure [1]. After considerable hesitation due to difficult anatomy and (patho-) physiology of the colorectum (table 1) and problems of stent design (table 2), stent implantation into the colon has been attempted relatively late.
Concerning pathology, there are very different tumor morphologies, sometimes with asymmetrical, even bizarre configurations of the stenosis – moreover, at this site, siphon and/or bend stenoses are often more pronounced than, e.g. in the esophagus or at the cardia. Extreme differences in lumen are another problem: Prestenotic distensions of the right colon of more than 10 cm in diameter have been repeatedly found. In such cases, the post-stenotic lumen often is extremely narrow. These circumstances, which are even subject to dynamic change after decompression, make effective and safe stent implantation quite difficult.
Even though SEMS for the colon are in the meantime available in a large variety of types, they rarely meet the requirements concerning configuration and diameter of tulip and shaft as well as flexibility and elasticity in an optimal way.
In this context stent implantation is used with favorable results [2] in order to avoid the need for a colostomy, which seriously compromises the quality of life of the often elderly patients. Nevertheless, it has to be taken into account that a palliative surgical resection should definitely be discussed for the colorectum as the only curative therapy.
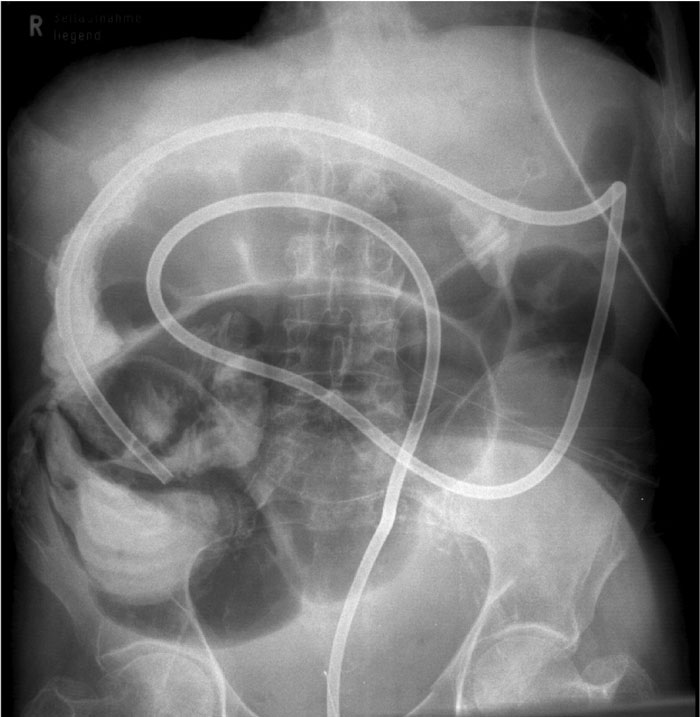
Figure 1: Correctly placed decompression tube immediately after insertion. A lot of gas and liquid stool passes.
To treat an acute tumor obstruction in the left colon, till now immediate emergency surgery has been the gold standard. After open laparotomy the intestine is subsequently decompressed and a colostomy is performed, which is associated with mortality rates up to 40% and complication rates up to 50% [3]. Afterwards several operations are necessary to resect the tumor and to close the stoma.
In such left-sided acute colon obstructions, stent implantation as a minimally invasive endoscopic method is discussed to induce decompression
and to bridge the interval to definitive surgery (“bridge to surgery”).
In the mid-1990s, the introduction of endoscopic procedures for primary decompression as a “bridge to surgery” constituted a decisive step forward. Decompression can be achieved by endoscopic placement of a decompression tube (see below) or by primary implantation of a stent.
Primary endoscopic decompression (fig. 1) is advisable and has the decisive advantage that emergency surgery with all its problems can be avoided and replaced by an elective operation after decompression and appropriate staging. This approach has a mortality of less than 5% and a morbidity of less than 15%, which is a huge improvement compared to the results of emergency surgery (see above); in most cases, it also avoids the need for a colostomy and reinterventions.
Beneath stent implantation, endoscopic placement of a decompression tube using the over-the-wire (OTW)-/Seldinger technique is possibly the easiest way to establish such a “bridge to surgery” (Table 3). This technique has the potential to meet all aims of the “bridge to surgery” concept. If placed correctly, the tube causes effective decompression very quickly, the patient is out of danger, and after intermittent staging, a differentiated therapeutic decision can be made without time pressure (e.g. elective surgery or targeted palliative therapy).
In case of absolute or relative inoperability (T4 tumor, metastatic disease, ASA III-IV etc.), the implantation of an SEMS instead of a decompression tube aims at reaching a definite – at least medium-term – solution of the problem with the first treatment step [2], but SEMS implantation in the acute situation with a distended colon represents a challenge, at least for technical reasons.
The first reports of stent implantations in the colorectum showed that such implantations are feasible and yield quite satisfactory results. Several years later, the results of metaanalyses [4] also led to very positive evaluations, and stents in the colorectum were described as “being on their way to becoming routine procedures”. However, a critical evaluation reveals that some of these studies report very short follow-up or survival periods (e.g. 42 days or 88 days) and describe cumulative complication rates of 30-70% as acceptable. Today, an analysis of the literature, which now comprises a substantial amount of studies, yields surprisingly contradictory results [5-12]. In the range of interventional endoscopy, there is probably no field with equally diverging results of clinical studies as that of stent implantation in the colorectum, particularly with regard to the indication “bridge to surgery”.
The fact that different patient groups and stent indications are mixed up to some extent has to be taken into account. However, emergency indications in the case of a manifest colon obstruction warrant a completely different assessment than elective indications for non- or scarcely stenosing colon carcinoma. Due to the serious impact of the pathophysiology of the mechanical ileus on complication rate, mortality and outcome, the two entities are not comparable.
Many studies deal with different time periods and different types of stents. Understandably, esophagus stents used in the early pioneering period for the colon are not comparable with especially developed colon stents.
Even if special colon stents are used, there are non-comparable stent types with different designs, complete, partial or no coating, with different mechanical properties and with different releasing systems.
Additionally, there are different implantation techniques – purely radiological, purely endoscopic, as well as combined endoscopic-radiological implantation. Moreover, there are different doctrines regarding the use, type, and extent of pretreatment (e.g. dilatation, argon plasma coagulation (APC)).
Furthermore, there is an obvious lack of comparability regarding the expertise of endoscopists; there are even some published studies in which the learning curve becomes obvious.
A main problem with the differences in study design is that the definitions of technical and clinical success rates as well as of the primary, secondary and tertiary endpoints of the studies are not identical. Moreover, the statistical analyses range from purely descriptive to highly complex [6,11].
There is a conspicuously low representation of randomized controlled studies carried out according to evidence-based medicine (EBM) criteria. For example, a recent meta-analysis [11] with 2844 analyzed studies showed that only four randomized controlled trials met the strict inclusion criteria, which corresponds to 0.14%. Since three of these four trials were stopped prematurely, partly for contradictory reasons (overwhelming superiority of stent implantation, on the other hand serious complications in the majority of patients in the stent group), only one single study is left for reference, which corresponds to 0.035% of the publications [11]. These data illustrate the enormous difficulty to perform a comparative evaluation of studies and study results in this field. The situation of non-comparable criteria of the different studies seems to be clearly underestimated even in latest publications [12].
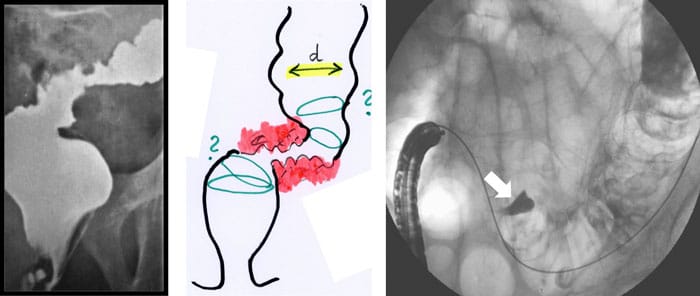
Figure 2: Planning a stent implantation according to configuration of the stenosis (a: X-ray, b: endoscopy). An intramural injection of contrast (Lipiodol®) exactly shows the target site (arrow).
Omission of staging and neglect of curative therapy options: The analysis of the literature shows, that the necessary staging is omitted to a high percentage (up to 50%), especially in stent implantations performed by radiologists (without endoscopy). After the symptoms of obstruction have been eliminated by stenting, patients are relatively free of complaints, and it is obviously difficult to motivate them to consent to undergo further steps for staging or even to consider surgical therapy, which constitutes the only curative option. Here, the “bridge to surgery” becomes a “bridge to wait and see”, which cannot be tolerated in view of the oncologic facts.
Spreading of tumor cells: For decades, oncologic (especially colorectal) surgery has followed the principle of ”no-touch technique” for operations [13], i.e. in order to avoid spreading of tumor cells or even perforation, mechanical alteration of the tumor should be kept to a minimum. These aspects should also be taken into consideration for endoscopic stenting measures, at least theoretically. According to experience during preparation and execution of stent implantation, it is impossible to avoid considerable mechanical irritation in the immediate vicinity of the tumor. Cave: any silent or overt perforation has to be seen as an oncologic catastrophe (tumor perforation is considered a T4-criterion!).
Combination with radio-/chemotherapy: All studies show that compared to an operation, stent implantation does not lead to a significant gain in time for the application of adjuvant therapy options (radiotherapy and/or chemotherapy). On the other hand, due to special situations in different health systems there are also sometimes long periods of waiting for an operation, also for the stent group.
Special attention should be paid to the fact, that several studies have reported a significantly higher perforation rate during or post stent implantation when
angiogenesis inhibitors (e.g. Bevacizumab) were used, with the serious consequences described above [12].
The expertise of the endoscopist has an important influence on the results: stenting in the colorectum is one of the most challenging indications in interventional endoscopy. The safest method seems to be a primarily endoscopic implantation in combination with fluoroscopy and use of contrast medium (fig. 2-5).
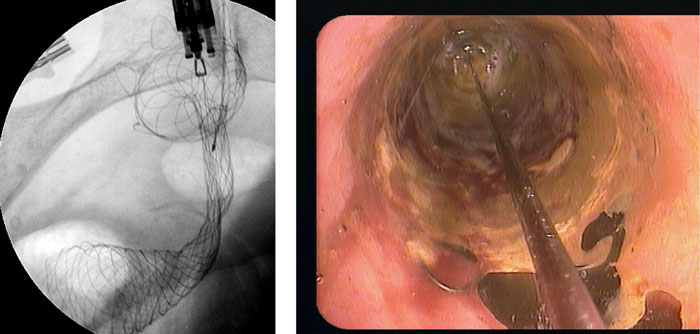
Figure 4: Insufficient stent deployment (a), treated by cautious balloon dilatation (trans-balloon view) (b).
The rectum, the left flexure and the right colon are particularly difficult. There are only few reports on successful stent implantation in the transverse colon, the right flexure, and the right colon. Most OTW stent delivery systems being too short for this localization, here even through-the-scope (TTS) systems need a highly experienced endoscopist [14]. On the other hand, due to tenesma and continence problems, implantation in the rectum is limited to especially suitable individual cases.
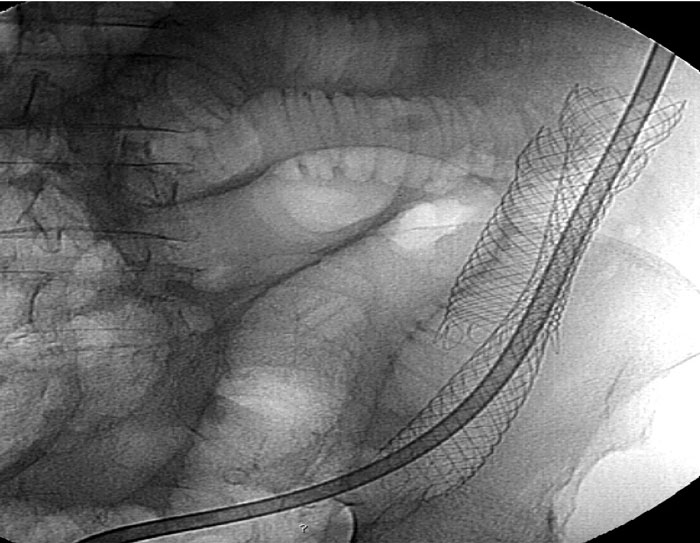
Figure 5: Secondary stent occlusion by lack of stool softeners after problematic SEMS implantation: Overstenting and intermittent decompression tube necessary.
The implantation of self-expanding metal stents (SEMS) into the colon is feasible and, in certain situations and for certain patients, it is also effective and helpful – especially as a “bridge to surgery” [2].
In cases of acute large bowel obstruction caused by a tumor in the left colon, such a procedure can reduce the serious problems and risks of emergency surgery, which would otherwise be necessary; after decompression the patient can undergo an elective operation if this is an option following the necessary staging and therapy planning. Other than in the esophagus, the bile ducts and the gastroduodenal junction, stent implantations in the colorectum are not routine measures, i.e. the indication has to be justified for each individual case. To date, no optimal stents are available for the colon, the high requirements for an appropriate prosthesis have only been fulfilled punctually and inadequately.
It also has to be taken into account that endoscopic placement of a decompression tube is an equally effective therapy option for decompression and can reach the same aim, a “bridge to surgery”.
Both alternatives for the “bridge to surgery” – decompression and stent implantation – require a very experienced endoscopist who is competent for the entire range of endoscopic interventions.
In general, oncologic aspects should always be respected. Radical surgery is the only curative treatment chance for patients with a colorectal carcinoma; it is mandatory not to gamble this chance by primary stent implantation without a further look into the curative option.
When evaluating the meanwhile extensive literature dealing with this topic, it becomes particularly clear that the comparability of studies represents a highly relevant and unsolved problem. Results in the literature have to be analyzed very critically in order to get helpful guidelines for clinical practice.